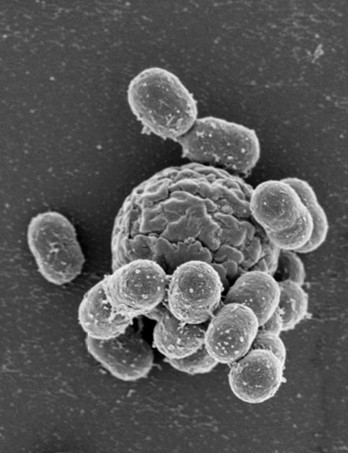
Gottfried WILHARM (Wernigerode, DE): The natural reservoirs of the nosocomial pathogen Acinetobacter baumannii are poorly defined. In previous work we identified white storks as a model system to study the ecology of A. baumannii. Having screened more than 1,200 white stork nestlings over a period of six years in different regions of Poland and Germany (overall isolation rate ~25%) and having included food chain analyses and environmental samplings, we come up with a detailed picture of the dynamics and diversity of A. baumannii in natural habitats. Adult storks, rather than being stably colonized with A. baumannii strains that are then transferred to their offspring, come in contact with A. baumannii while foraging. Among their prevailing food sources consisting of earthworms, mice, and insects, we identified earthworms as a potential source of A. baumannii, but also the associated soil as well as plant roots. Specific soil and compost habitats were identified that allow population dynamics to be studied over the course of the year. The prevalence of A. baumannii exhibited a strong seasonality and peaked in summer. De-composition of plant material under fungal regime appears to set the stage for proliferation of A. baumannii. The diversity found reached up to 10 distinct lineages isolated from a single sample site on a single day. Exten-sive horizontal gene transfer was detected between co-colonizing lineages. Over a season, more than 20 dis-tinct lineages could be isolated from individual sample sites. The strains we collected in Poland and Germany represent more than 60% of the worldwide known diversity in terms of the intrinsic OXA-51-like β-lactamase. Core-genome based phylogenetic analyses illustrate numerous linkages between wildlife isolates and hospital strains, with the respective last common ancestors dating back hundreds to thousands of years.
Our data suggest a persistent worldwide spread of A. baumannii lineages since ancient times. Linking pub-lished work on the interaction between A. baumannii and fungi, on aspergillosis as a major cause of mortality in white stork nestlings, and our findings, we hypothesize that fungi and A. baumannii share a long history of co-evolution. Interaction studies revealed the capability of A. baumannii to adhere to fungal spores and suppress spore germination. Taken together, the intrinsic resistance endowment and potential to acquire antibiotic re-sistance can be explained by coevolution with antibiotics-producing fungi, and resistance to desiccation stress and radiation can be interpreted in the light of hitchhiking on fungal spores.